Sina Shisheh Darman (SSD group) stands at the forefront of glass pharmaceutical packaging innovation
Sina Shisheh Darman (SSD group) adheres to stringent quality standards by employing a certified quality management system in its production facilities. This system ensures the consistent application of GMP requirements specifically tailored for pharmaceutical primary packaging.
This revised version emphasizes the company’s commitment to quality by highlighting the use of a certified quality management system and the consistent application of GMP requirements. It also clarifies that these requirements are specifically tailored for pharmaceutical primary packaging.
PRODUCTS





Manufacturing Process
WE KNOW GLASS
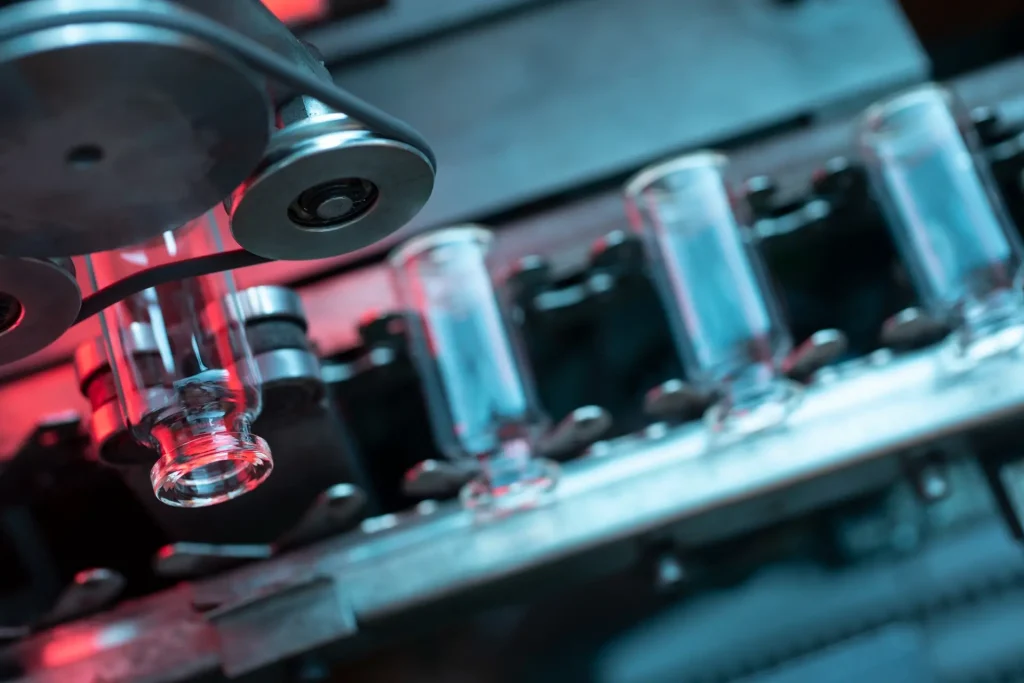
SSD group vials meet a wide range of requirements depending on precision instruments and processes. It can satisfy the needs of the most demanding pharmaceutical company requirements on resistance, cosmetics, and dimensions through continuous monitoring of all critical parameters that influence the forming process.

Continuous Tube Loading

Neck Forming

Dimensional And Cosmetic Inspection

Screw/Crimp Forming